The many-faced macrophage
Macrophages may have opposing roles in tumor progression. As part of the innate immune system, macrophages survey an organism’s biological landscape and quickly engulf foreign bodies for disposal. When exposed to unfamiliar cell surface antigens on cancer cells, macrophages may follow a classical activation pattern (M1 macrophages) and initiate anti-tumor responses. Cancer cells, however, can disguise or alter their cell surface markers, convincing macrophages to recognize them as “self” and ensure their survival. Alternatively, under more sinister conditions, cancer cells may recruit and activate macrophages as accomplices to promote their survival (becoming M2 macrophages). Recent research indicates that the majority of tumor-associated macrophages (TAMs) assume this latter pro-tumoral role. In a study published in the journal Neoplasia in April 2015, researchers at MD Anderson Cancer Center in Houston, Texas characterized and eliminated TAMs in a mouse model of glioma, and demonstrated that TAM identity can change upon baseline reconstitution (Gabrusiewicz et al 2015).
The MaFIA mouse hitman, AP20187, eliminates M2 macrophages and reduces glioma burden
To understand the role of macrophages in tumor progression, Gabruiewicz and colleagues utilized the transgenic Macrophage Fas-Induced Apoptosis (MaFIA) mouse model for their studies (C57BL/6-Tg(Csf1r-EGFP-NGFR/FKBP1A/TNFRSF6)2Bck/J, 005070). In MaFIA mice, Csf1r-expressing macrophages are labeled with GFP. Further, these macrophages can be transiently depleted by in vivo administration of a compound, AP20187, which induces apoptosis.
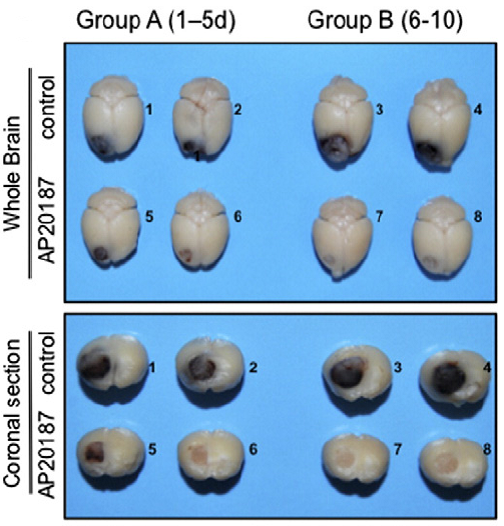
Using the MaFIA model, the MD Anderson team injected syngeneic GL261 glioma cells into one side of the animal’s brain where they formed robust tumors within 2 weeks. Immunofluorescence analysis revealed a large influx of activated, GFP-labeled, pro-tumoral M2 TAMs both at the tumor periphery and in the center. Upon administering AP20187, only the M2 TAMs were reduced; normal, endogenous macrophages on the uninjected, contralateral side of the brain were unaffected, likely due to the inability of the drug to cross the intact blood-brain-barrier. Surprisingly, M2 TAM reduction in the tumor was followed by an increase in anti-tumoral M1 TAMs. In addition, AP20187 treatment resulted in stark and visible reduction of tumor burden (Figure 1), as well as decreased tumor cell proliferation and blood vessel density in tumor-bearing tissues. Together, these results indicate that M2 TAMs support tumor progression, while M1 TAMs may not. Whether or not rebounding macrophages will default to the M1 phenotype and will directly eliminate cancer cells in a spontaneous tumor model warrants further investigation.
These results add to the growing literature that macrophages are influential characters in the tumor microenvironment. Macrophages, among other tumor-associated leukocytes, have the dual ability either to benefit the tumor or to be therapeutically manipulated to benefit the patient. Finding treatments that inhibit the former and stimulate the latter may contribute to important, new cancer therapies.