Most cancer deaths result not from primary tumor growth, but from the subsequent spread of cancer within the body, a process called metastasis. Few of the cells that break away from the tumor are able to survive the trip through the bloodstream and eventual colonization of a new organ or tissue, but specific environments, such as the lung, are relatively receptive to them.
Jackson Laboratory (JAX) Assistant Professor Guangwen “Gary” Ren, Ph.D., investigates the mechanisms that underlie metastatic tumor growth in distant organs and identifies potential molecular targets for both preventative and treatment interventions.
Two recent papers from Ren and his team have focused on different aspects of mesenchymal cells (MCs) in the lung, and how they can assist lung metastasis by breast tumor cells. MCs, a majority of which are fibroblasts, are usually associated with formation of the structural framework for different kinds of tissues and are essential for tissue regeneration upon injury. In the lung, MCs play key regulatory roles during early lung development and maintain lung stability in varying conditions. However, the roles of MCs in lung metastasis of solid cancers are less well characterized. The new studies showed that MCs can promote immunosuppression within the lung and provide metastatic cells with fuel in the form of lipids, both of which help tumor cells survive and grow to form successful metastasis.
Misdirected lipids
The first paper, published in Cell Metabolism, detailed how Ren and collaborators, including Professor Lenny Shultz, Ph.D. and Professor/President Emeritus Edison Liu, M.D., used breast cancer models to determine how lung MCs become loaded with lipids. The paper, titled “Lipid-laden lung mesenchymal cells foster breast cancer metastasis via metabolic reprogramming of tumor cells and natural killer cells,” reveals that the lipid accumulation in lung MCs is the result of a molecular pathway controlled by IL-1ß signaling. The lipid-laden MCs transport their lipids to tumor cells, leading to heightened survival, as well as natural killer (NK) cells, causing dysfunction. The combination helps facilitate breast cancer lung metastasis, and blocking IL-1ß improved the efficacy of NK cell-based immunotherapy.
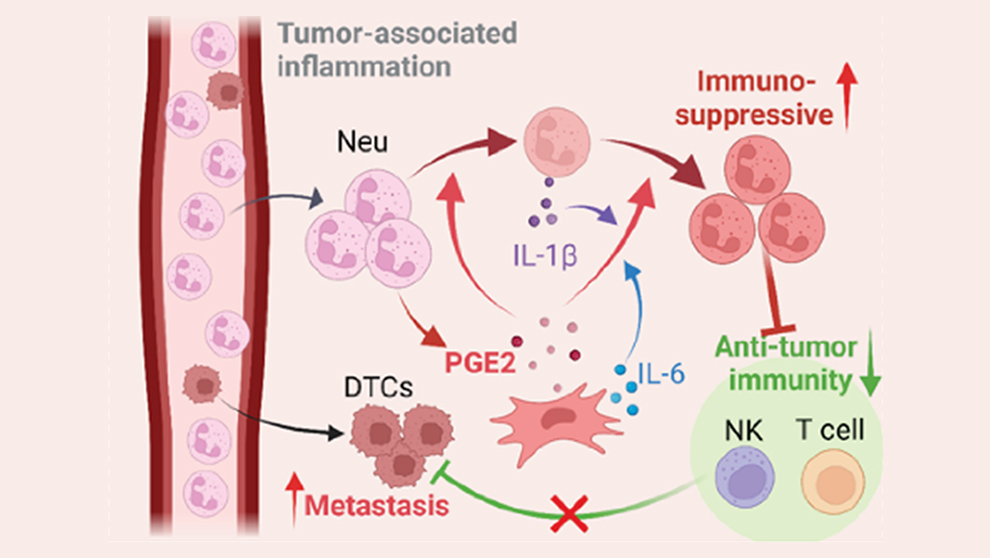
Immunosuppressive neutrophils
In related work, Ren, Shultz and colleagues investigated another way mesenchymal cells make lung tissue more receptive to tumor cells: immunosuppression. In this case, a particular subset of lung MCs boost the immunosuppressive activity of neutrophils, an important type of immune cell. Neutrophils are the most abundant innate immune cell, usually serving as the front line of defense against pathogenic microbes. But in cancer, neutrophils have been shown to actually promote cancer growth and suppress anti-tumor immune responses, though the mechanisms involved within metastatic microenvironments are not yet well understood. In “Immunosuppressive reprogramming of neutrophils by lung mesenchymal cells promotes breast cancer metastasis,” just published in Science Immunology, Ren and his team use specialized mouse models as well as human cell cultures to show that mesenchymal cells underlie neutrophil-promoted immunosuppression within the lung and provide invading cancer cells with a better chance of survival.
The team first determined whether neutrophils have immunosuppressive characteristics in other tissues, or if it’s specific to the lung. When isolated from spleen, bone marrow or blood, neutrophils were found to be far less immunosuppressive than those isolated from the lung. And in the lung, they exhibited immunosuppressive qualities both in the presence of tumors and in tumor-free naïve mice. Interestingly, when bone marrow-derived neutrophils were introduced into lung and other tissues, only those in the lung substantially increased their expression of immunosuppression-associated genes, indicating the presence of a lung-specific reprogramming mechanism.
Mesenchymal cell influence
What is it in the lung, then, that drives the reprogramming of neutrophils to a strongly immunosuppressive state? The researchers cultured lung stromal cells with bone marrow-derived neutrophils to see which cell subset promoted the change. They identified a particular type of MC, known as CD140a+ (mostly fibroblasts), that significantly increased neutrophil expression of immunosuppression-associated genes. Although present in other tissues, the lung-resident CD140a+ cells were found to have the most prominent effect. The reprogramming is not dependent on MC-neutrophil contact, so the team hypothesized that a soluble factor induces it through a signaling pathway. Investigating further, they identified prostaglandin E2 (PGE2), an inflammatory lipid mediator produced by lung MCs, as the primary molecule responsible.
When in an immunosuppressive state, the neutrophils inhibit the anti-tumor cell activity of T cells and NK cells, also immune cells that normally detect and eliminate cancer cells that have separated from the primary tumor. If the neutrophils’ immunosuppression was blocked, however, would the typical surveillance and elimination immune functions resume and prevent metastasis? Using mice engineered with PGE2 deficiency, the team found that lung T cell proliferation and NK cell function were not suppressed nearly as much as in wild-type mice, leading to lower levels of lung metastasis. They then pharmacologically blocked EP2 and EP4, two PGE2 receptors, to inhibit PGE2 signaling, and observed similar effects.
Treating cancer metastasis in the lung
The findings have important implications for therapy development to reduce the incidence of metastatic cancer in the lung. Immunotherapies often involve engineering or activating T cells to attack cancer cells, but such strategies are far less effective in an immunosuppressive environment, and the researchers confirmed that one such treatment, adoptive T cell transfer therapy, had a loss of therapeutic efficacy in treating lung metastases. Blocking the PGE2 receptors as above enabled the effectiveness of the therapy, however, indicating the potential for an effective combination therapy for treating lethal lung metastasis.