
JAX scientist Max Presa meets with colleagues to discuss research progress into Multiple Sulfatase Deficiency. Photo by Tiffany Laufer.
Recently, the Accelerating Medicines Partnership Bespoke Gene Therapy Consortium (AMP BGTC), a public-private partnership spanning NIH institutes and centers, life sciences companies, and nonprofit organizations was formed, with the goal to catalyze progress toward first-in-human gene therapy clinical trials for rare disease patients. This new consortium will positively impact rare disease patients and families, and is a powerful and gratifying next step for those gene therapies that have shown so much promise in preclinical experiments. Reinforcing the important work of Cat Lutz, Ph.D., MBA, and the Rare Disease Translational Center (RDTC) team and researchers including Rob Burgess, Ph.D., we are today seeing the fulfillment of the next step in accelerating the production and delivery of effective therapies for those in the rare disease community.
Our work has been pivotal to creating the foundation upon which AMP-BGTC will build a faster and more efficient pathway to therapeutics. Over the past five years, the JAX team created mouse models and conducted the pivotal preclinical work on three of the eight diseases selected for AMP-BGTC clinical trials. Translating these genetic discoveries to the clinic exemplifies the contribution of JAX to improving human health, the ultimate aim of many basic scientists worldwide. Scaling this process to more diseases and translating it more rapidly is a cornerstone objective of our RDTC and a proof point on JAX’s unique position within rare disease research.
Below is additional information on the new consortium and the three diseases for which JAX’s Rare Disease Translational Center made fundamental contributions. All three illustrate the unique and critical role JAX can play in working with families, foundations, and other members of the rare disease community, as well as the vital importance of our expertise in model development in accelerating discovery and translation to the clinic.
Implementing gene therapies for rare diseases
The Accelerating Medicines Partnership Bespoke Gene Therapy Consortium (AMP BGTC) is a public-private partnership spanning NIH institutes and centers, life sciences companies, and nonprofit organizations. As its name implies, the consortium’s goal is to catalyze progress toward first-in-human gene therapy clinical trials for rare disease patients. In 2021, the consortium launched a rare disease selection process for its clinical trial portfolio, and on May 16, they announced the eight diseases chosen, which include Multiple Sulfatase Deficiency (MSD), Charcot-Marie-Tooth disease type 4J (CMT4J), and spastic paraplegia 50 (SPG50).
Multiple Sulfatase Deficiency
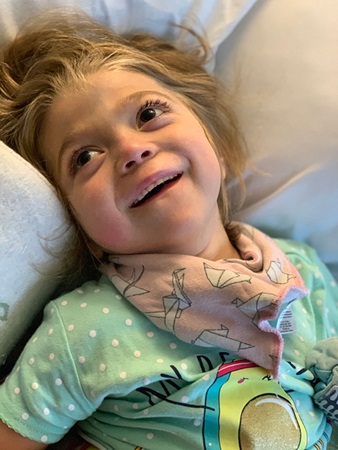
MSD is caused by mutations in the SUMF1 gene, which codes for a protein necessary for breaking down cellular waste products. Dysfunction leads to neurodegeneration and premature death, usually before 10 years of age. Years of work by Cat Lutz and her long-term collaborator Steven Gray at the UT Southwestern Medical Center was inspired by Amber Olsen and Alan Fingus, both of whom have children with this disease. A gene therapy strategy in mice showed that using an engineered virus to deliver working copies of the SUMF1 gene at seven days of age alleviated MSD symptoms with no signs of adverse side effects. These promising results were the inspiration for the Olsen and Fingus families’ foundations to aggressively pursue gene replacement therapy to treat their children. According to Cat Lutz, “Summing up the experimental results does not begin to capture our longstanding relationships with Amber, Alan and research colleagues around the world. Max Presa at JAX led the experiments and years of meetings to strategize, rethink, and develop the best data we could to support multiple therapeutic programs for MSD.”
"We are very excited by the news of our research partners at Children's Hospital of Philadelphia receiving a Bespoke Gene Therapy Consortium grant. This funding will move MSD gene therapy research into clinical trials," stated Sarah Cortell Vandersypen, United MSD Foundation's Executive Director. "We are very grateful to everyone whose support has helped get MSD AAV9 gene therapy to this point, especially The Jackson Laboratory, who have been invaluable colleagues over the past five years. The mouse study conducted under their purview showed that curing MSD is possible, providing hope to so many MSD families.”
Charcot-Marie-Tooth disease type 4J
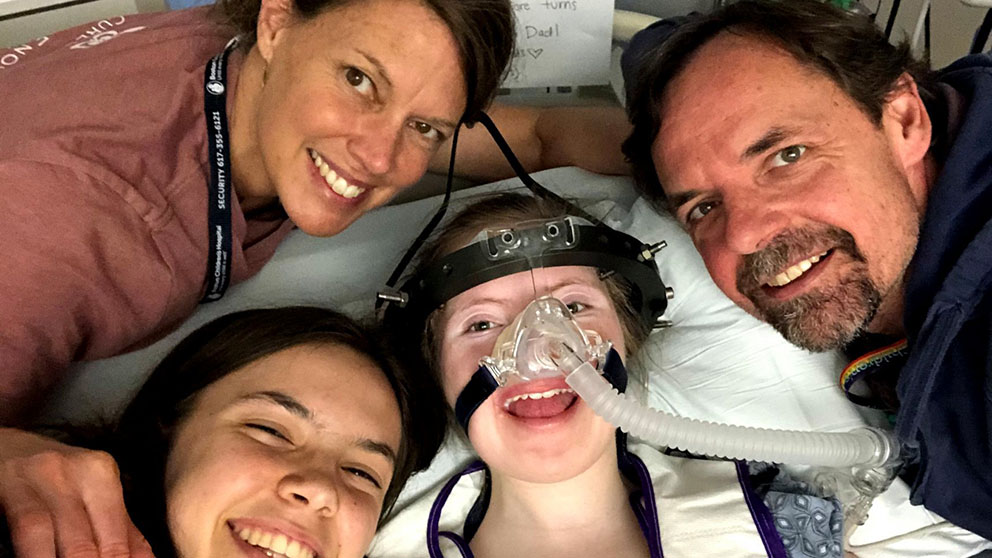 Talia Duff (center) with her family. Talia has Charcot Marie Tooth Type 4J and her family founded Cure CMT4J. Photo provided by Cure CMT4J.
Talia Duff (center) with her family. Talia has Charcot Marie Tooth Type 4J and her family founded Cure CMT4J. Photo provided by Cure CMT4J.
Loss-of-function mutations in both copies of the FIG4 gene lead to CMT4J, characterized by progressive loss of sensory and motor neuron function, though the exact symptoms and severity are highly variable. Using a mouse model of CMT4J that exhibits disease symptoms similar to that in humans, JAX RDTC scientists used viral delivery of an optimized wild-type human FIG4 gene at birth or soon thereafter to restore gene expression and function. The treatment lengthened survival from approximately thirty five days to over one year, and alleviated disease symptoms in a remarkable fashion. Jocelyn Duff is the founder of the CureCMT4J Foundation and first met with Drs. Cat Lutz and Steven Gray in a “call to arms” meeting in 2016; JAX has been supporting her efforts to get this therapy to the clinic ever since.
"Our inclusion in the BGTC platform could not have been possible without the expertise of the JAX team," says Duff, whose daughter, Talia, has CMT4J. "The BGTC was looking for rock solid preclinical proof of concept and sound science to back it up. CureCMT4J's work with JAX has provided not only that, but the compassion and urgency that Cat Lutz, Rob Burgess and Max Presa bring to all of their work in rare disease."
“Max Presa, Ph.D. was the lead scientist in the RDTC who generated all of the preclinical data supporting the efficacy of the gene therapy approach for CMT4J that has now been picked up by the BGTC,” said Professor Robert Burgess, Ph.D., CMT researcher and co-author on https://www.jci.org/articles/view/137159, the paper that demonstrated the efficacy of gene therapy for CMT4J.
Spastic paraplegia 50
 The Pirovolakis family and their son, Michael, who was diagnosed with SPG50 at 15 months old. Photo provided by Terry Pirovolakis.
The Pirovolakis family and their son, Michael, who was diagnosed with SPG50 at 15 months old. Photo provided by Terry Pirovolakis.
JAX developed a mouse model for SPG50, a rare disease caused by mutations in AP4M1, which produces a protein involved with correctly locating other proteins within cells. Individuals with SPG50 are usually non-verbal, unable to walk, and have significant cognitive impairment. Working with CureSPG50, an organization established by Terry Pirovolakis, father to Michael, who was diagnosed with SPG50, Lutz and her team helped organize the mouse efforts that were needed to advance preclinical gene therapy strategies for SPG50. Lutz and Pirovolakis remain connected today in an effort to help more patients with rare disease. “Terry is an amazing individual who has not only successfully treated his son, but has dedicated his experience and business model to helping other rare patients,” says Lutz. Pirovolakis’s company, Elpida Therapeutics, is playing a pivotal role in the Bespoke program.