Clinically, Alzheimer’s disease (AD) is a disease that is diagnosed largely through indirect indicators, especially in early stages. For decades, any definitive diagnosis had to be posthumous. Now, various cognitive tests and AD-associated protein measurements in cerebrospinal fluid provide reasonably solid diagnostic indicators that can be further supported by brain scans using advanced imaging technologies.
But why is AD so difficult to diagnose rapidly and precisely? One reason is that outside of protein buildup in the brain, primarily beta-amyloid plaques and tau tangles, many of the key biological processes involved with AD development and progression remain unclear. Also, research has recently indicated that AD likely has distinct, genetically defined subtypes. Finally, it has become apparent that different people experience variation in disease timing and severity, even when they have the same rare, high-risk genetic mutations, suggesting that background genetics plays an important role in AD pathology.
Using mouse models for AD with diverse genetic backgrounds, a research team led by The Jackson Laboratory (JAX) Assistant Professor Erik Bloss, Ph.D., and Professor Gareth Howell, Ph.D., explored how different genetic contexts affect the impact of early-stage AD on neuronal circuits. The team, which also included first author Sarah Heuer, a predoctoral associate in the Howell and Bloss labs, demonstrated that synaptic alterations in a specific AD-vulnerable neuronal projection pathway are, in fact, differentially controlled by genetic context. The results were presented in “Control of hippocampal synaptic plasticity by microglia-dendrite interactions depends on genetic context in mouse models of Alzheimer’s disease” — a paper published in Alzheimer’s & Dementia, the journal of the Alzheimer’s Association.
A resilient mouse
Most research into the basic biology of AD has been conducted using an inbred mouse strain called C57BL/6J, or B6 for short. While highly useful for studying AD pathology in one genetic background, working with one mouse strain doesn’t provide answers regarding why different people with the same genetic mutations or AD risk factors exhibit variable disease manifestations. The research team therefore added a wild-derived mouse strain, known as PWK/PhJ (PWK), which has a markedly different genetic background to B6. Previously, the researchers had shown that PWK mice with mutations in two key humanized AD-related genes, APP and PSEN1 (PWK.APP/PS1), and the commonly studied B6.APP/PS1 mice, had identical beta-amyloid plaque deposition. Nonetheless, the PWK.APP/PS1 mice had notably greater cognitive resilience than their B6 counterparts.
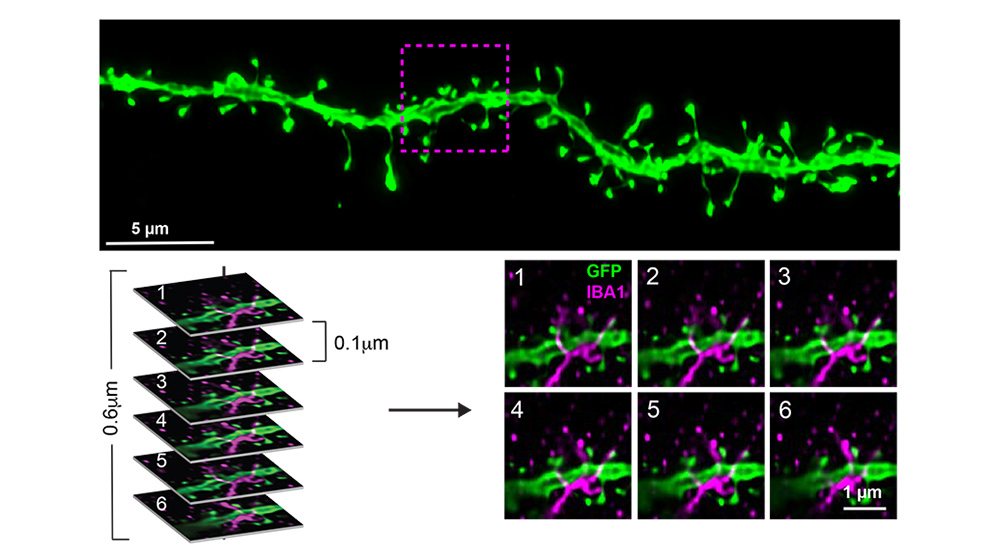
To investigate what could be causing these cognitive differences, the researchers focused on a specific AD-vulnerable neuronal circuit that connects a region of the hippocampus to the prefrontal cortex. Given that activation of microglia — brain-specific immune cells that help maintain neuronal networks and repair injury — is implicated in AD, they used a dietary supplement to deplete the microglia in half of each group of mice (B6; B6.APP/PS1; PWK; PWK.APP/PS1), while the other half of each group was fed a control diet. The microglia depletion did not alter beta-amyloid plaque deposition in any of the mice.
The researchers then looked at the spines of neuronal dendrites, which are the structures that form synapses to transmit and receive signals from other neurons. Measuring dendritic spine density provides an approximation for synaptic number, while spine morphology (structure) indicates synaptic stability and strength. They found that microglia-synapse interactions in healthy and beta-amyloid-exposed brains depend on genetic context. B6 synapses in the presence of beta-amyloid plaques exhibited smaller spine size (less stable), while in the absence of microglia they were larger (more stable). These effects were absent in PWK mice, suggesting that PWK synapses exhibit a form of resilience to beta-amyloid plaque pathology. Additionally, the changes in spine density in B6 mice were dependent on microglia being in direct contact with the dendritic branch. This relationship was absent in PWK microglia-dendrite interactions, further confirming the synaptic resilience exhibited by these mice is not driven by microglia.
Genetic protection?
While much research has focused on why some people are at a higher risk for AD, there has been a recent focus on protective mechanisms. Some can be behavioral/environmental, such as the lower risks that have been associated with consistent aerobic exercise. But others appear to be genetic, preventing or delaying onset of disease in individuals with typically deterministic mutations. But more work is needed to ascertain exactly what molecular mechanisms serve to protect these people, as well as resilient mouse strains like PWK, and whether they can be targeted and boosted with preventative treatments to help individuals that are more susceptible.
“Based on the results, we nominate the PWK/PhJ mouse strain as a model of resilience to AD, both at the synaptic and cognitive level,” says Heuer. “While microglia depletion didn’t impact PWK synaptic responses in our AD models, we are now conducting additional studies to identify other cell type-specific drivers of PWK resilience.”