
Many strains expressing Cre have phenotypes that were not obvious or characterized when the mice were created. Some of these phenotypes, including unexpected expression patterns and unpredictable deletion efficiencies may seriously complicate the interpretation of your experiments as we explained in our previous blog post, Cre-lox myths busted. Here we describe in more depth some of the problems you may face and how to work around them.
Off-target Cre expression
Off-target Cre expression occurs when Cre is expressed in tissues or organs not directly dictated by the promoter used to drive Cre expression. If this happens with your cre strain and you are unaware of it, then your floxed gene may be deleted in unexpected tissues. This can present you with potential problems, mainly with interpreting your data inaccurately.
Therefore, make sure that you are well-informed about the expression pattern of your cre strain(s). This starts with reviewing the published literature to see which tissues and organs have been examined for Cre expression. When investigators generate new cre strains, they, of course, look for Cre expression in the tissues where the promoter should drive it, but they often don’t verify the absence of Cre expression in the other non-target tissues.
If Cre expression information is not available for your strain, then you should cross your cre strain to one of numerous Cre reporter strains that express a visible marker, such as b-galactosidase, alkaline phosphatase, luciferase, GFP or another fluorescent protein, in cells where Cre is expressed. Only a single cross is needed to generate mice that carry both the cre transgene and the Cre reporter, which is all that is required to check that Cre is expressed in just the cells you want.
Off-target Cre expression in the Germline
Off-target Cre expression in the germline can be particularly troublesome, because you might generate mice in your colony with a fully recombined null allele in all of their cells. Frequently, germline expression of Cre is due to the post-meiotic persistence of the cre RNA or protein in oocytes. For many cre strains, but not all, using cre-positive males for breeding avoids potential germline deletion of your loxP-flanked allele. You can easily monitor colonies for germline expression of Cre via PCR by designing primers that anneal upstream and downstream of your floxed exon(s). That way, if a complete, recombined knockout allele is generated, you will amplify the smaller product (Figure 1). Genotype all of your breeders and remove any that show the deleted allele from your colony.
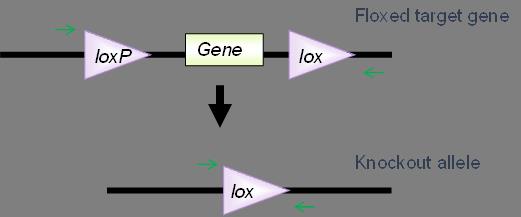
LoxP site recombination inefficiency
The efficiency with which Cre recombines loxP sites is affected by their genomic position and the distance between them. Therefore, the same cre transgene can cut different loxP-flanked alleles with different efficiencies. Finding out that your loxP-flanked gene is not cut efficiently (that is, that gene expression from your floxed target is not sufficiently reduced) can be extremely disheartening.
-
Step 1
If you do find yourself in this situation, you should re-genotype your mice (with a new DNA sample) to confirm that your mice are homozygous for the loxP-flanked allele AND carry the cre transgene.
-
Step 2
Next, review your methods for testing deletion efficiency/gene expression and make sure that they are appropriate for your specific loxP-flanked allele. For example, if the conditional allele has loxP sites flanking exon 5 of your gene, then following recombination, exon 5 is deleted, but exons 1-4 and the promoter remain intact, and a transcription product derived from the recombined locus may still be produced. If your primers for monitoring mRNA expression fall upstream of exon 5, you then may not see a significant decrease in expression.
-
Step 3
If you confirm that Cre is not cutting your loxP-flanked gene efficiently, then try introducing a complete knockout allele of your gene into the colony, and generate mice that are compound heterozygotes (one loxP-flanked allele and one null allele) for your target gene and that carry the cre transgene. In these mice, Cre only has to recombine one loxP-flanked allele, not two, which may improve its recombination efficiency.
Strains with tamoxifen inducible Cre
If your cre strain is tamoxifen inducible, then follow steps 1 and 2 described above. Next, cross your tamoxifen-inducible cre strain to a Cre reporter strain. Test your tamoxifen administration protocol on the cre-positive, reporter-positive mice to confirm that tamoxifen is reaching your tissue/organ of interest and efficiently inducing recombination. In fact, it is a good idea to do this at the beginning to work out your tamoxifen-induction protocol ahead of time. The following link provides an overview of several different routes of tamoxifen administration, including the pros and cons of each;
http://openwetware.org/wiki/Tamoxifen_administration_to_mice. If all these steps check out, then perhaps introducing a complete null allele as described above (step 3) may improve the tamoxifen-dependent recombination efficiency.
Unsolicited Cre phenotype
Cre may produce a phenotype all by itself, which can complicate interpreting your data. This can be caused by cre transgene insertion site effects on nearby, endogenous genes. Also, mice expressing high levels of Cre may develop mutant phenotypes or mortality due to "Cre toxicity" caused by recombination between cryptic semi-homologous loxP sites in the mouse genome. In order to make sure that any phenotypes you observe are due to deletion of your gene and not due to the cre transgene, include cre-positive mice that are wild-type for your loxP-flanked allele as controls in your experiment.
Before you generate your next conditional knock-out model, you should familiarize yourself with the pattern of Cre expression in the strain you will be using. The JAX Cre resource has characterized Cre expression patterns in over 110 Cre-expressing strains in the JAX® Mice Repository, with more data to come. Be an informed scientist and check it out! If Cre expression data for a particular strain is not publicly available, we really encourage you to investigate it for yourself to avoid major headaches in the long run.