Rita Levi-Montalcini was born on April 22, 1909, in Turin, Italy. Rita and her twin sister Paola were the youngest of four children born to Adamo Levi and Adele Montalcini. Her mother was a painter, and her father was a mathematician and electrical engineer; both came from Jewish families whose roots extended back to the Roman Empire. In the post-Victorian era, men were considered the head of the family, while women were generally confined to roles in the home. Adamo was staunchly against his daughters attending university, as it would interfere with their primary roles as wife and mother.
At age 20, Levi-Montalcini decided that she wanted a life different from the one imagined for her by her father; specifically, she wanted to go to medical school and study to be a doctor. She explained in her 1988 autobiography, In Praise of Imperfection, “My experience in childhood and adolescence of the subordinate role played by the female in a society run entirely by men, had convinced me that I was not cut out to be a wife.”
With her mother’s encouragement, Levi-Montalcini approached Adamo to tell him about her desire to study medicine. “He objected that it was a long and difficult course of study, unsuitable for a woman. Since I had finished school three years previously, it would not be easy to take it up again. I assured him that I was not afraid of that.” She made up her coursework in the span of eight months, and began studying at the Turin School of Medicine in 1930.
While at Turin she worked under the tutelage of Giuseppe Levi (no relation to Levi-Montalcini), a renowned Italian histologist that made a great impact upon the work ethic and scientific curiosity of his students. During her time in Levi’s lab she became enamored with the process of neurogenesis. Nerve cells, or neurons, are elongated cells that originate in the spinal column during development. Part of the nerve cell, the axon, extends outward from the spinal column and migrates to its final destination in various peripheral organs and tissues. The axon receives signals in these tissues that are transmitted back to the spinal cord and brain. At the time it was unknown how neurons determine their final location, and the different processes that govern their proliferation, differentiation, and survival. The skills Levi-Montalcini perfected in Levi’s lab would, in time, provide an answer to many of these pressing questions.
Levi-Montalcini graduated summa cum laude from the Turin School of Medicine in 1936 and began a three-year fellowship in neurology and psychiatry, while continuing her research on the development of nerve cells. Within this time frame it became increasingly dangerous to be a Jewish citizen in Europe. Mussolini came into power in 1922, and in 1938 he enacted the Manifesto per la Difesa della Razza, or the Manifesto of the Racial Scientist. In agreement with Hitler’s anti-Semitic views, these racial laws declared that “pure” Italians were descendants of the Aryan race. This policy was a justification of stricter laws to come, and later in 1938 a set of Racial Laws, or the Leggi razziali, were enacted to further strip Jewish citizens of their civil rights.
In 1939 she made the difficult decision to end her work at the University of Turin, not wanting to endanger colleagues by their association with a Jewish scientist. She continued her research in Belgium, where she had received an invitation to perform research at a neurological institute. But this respite was not to last. As Hitler’s influence in Europe spread, she feared for her family, and returned home to Turin in early 1940.
Even with the world coming apart around her, Levi-Montalcini was determined to continue her research. After returning to Turin, Levi-Montalcini set up a small laboratory in her bedroom, complete with a microtome and microscope for studying neurogenesis in the chick embryo. The bombing in Turin intensified in 1941, forcing her family to move to the countryside. Undeterred, she packed up her equipment and set up her bedroom lab a second time.

Levi-Montalcini was intrigued by a 1934 paper from Viktor Hamburger, in which he tested the requirement of different tissues for development and migration of nerve cells destined for this same tissue. The chicken embryo is an excellent model system for these experiments because it has a very consistent pattern of neuronal migration, so that sensory neurons can be observed at each stage as they extend to their final destination in peripheral tissues. Hamburger found that loss of the wing bud resulted in smaller and fewer nerve cells growing out from the spinal column, and came to the conclusion that the limb bud contained an organizing factor required for nerve cell growth, development, and innervation of the wing.
Levi-Montalcini was curious, and wanted to look more closely at these nerve cells in limb-bud deficient chick embryos and healthy embryos. She used only the equipment in her home laboratory, and convinced neighboring farms to sell her fertilized chicken eggs for her research. The process was meticulous, as she sectioned and stained chick embryos at each stage of development, with or without the wing bud removed, monitoring the development of sensory neurons.
She discovered something entirely novel.
Contrary to what she expected, a normal number of neurons were migrating towards the absent wing bud in the mutant embryos. It was later in development that a large number of these neurons died, resulting in the neuronal hypoplasia observed by Hamburger. In addition, she noted a significant amount of cell death in healthy embryos, suggesting that cell death was a normal part of neuronal development.
Levi-Montalcini came to a conclusion distinct from that of Hamburger. Instead of a peripheral organizer that promoted growth of neuronal cells, Levi-Montalcini concluded that the limb bud produced a pro-survival factor, which an overabundance of developing neurons compete for to survive and innervate the developing wing. Neurons that fail to make strong connections, and do not innervate, die as a normal part of neural development.
It was next to impossible for her to publish in academic journals in Italy during WWII. With the help of her former advisor, Giuseppe Levi, she sent manuscripts to Belgium and published her results in 1942 and 1943. In the fall of 1943 she and her family were forced to move again, this time to Florence, where they remained underground until August 1944. After American troops forced the Germans out of Florence, she worked as a medical doctor, helping treat refugees until the end of the war in 1945.
During this time Hamburger took a great interest in Levi-Montalcini’s work, and asked her to visit his lab at Washington University in St. Louis, MO. In 1947, she traveled to the United States and began her collaborations with Hamburger’s lab. Although she was only meant to stay a semester, she ultimately spent 30 years at Washington University, becoming a Full Professor in 1958, and holding this position until her retirement in 1977. In 1962 Levi-Montalcini established a second lab in Rome, and split her time between the U.S. and Italy. In 1969 she became the first Director of the Institute of Cell Biology of the Italian National Council of Research, also in Rome. She served as director until her retirement in 1979, and then continued on as a Guest Professor.
One of the key discoveries she made during her time in the United States was developing an in-vitro culture technique that allowed her to grow neurons in a dish, outside of the embryo. It began with the observation that a mouse tumor cell line caused increased nerve cell growth. When grafted onto a chicken embryo, these cancer cells attracted and stimulated neuronal growth, suggesting that these cancer cells contained the presumptive pro-survival, or “trophic,” factor. Levi-Montalcini visited Herta Meyer’s lab at the University of Brazil in the early 1950s, where she developed techniques to culture nerve cells and characterize factors that promote neuronal growth.
In addition, Hamburger recruited a talented young biochemist at Washington University, Stanley Cohen, to assist in the molecular characterization of the trophic factor. Cohen suggested using nucleic acid inhibitors to determine if the trophic factor had an essential DNA or RNA component. A crucial experiment took advantage of snake venom, which was known to degrade both RNA and DNA. The control experiment revealed that the venom itself actually contained potent amounts of the pro-survival factor, indicating that submaxillary glands could be used as a source to isolate and purify the unknown factor. Indeed, mouse submaxillary glands were a rich source of this factor, which provided ample resources for experiments and molecular characterization.
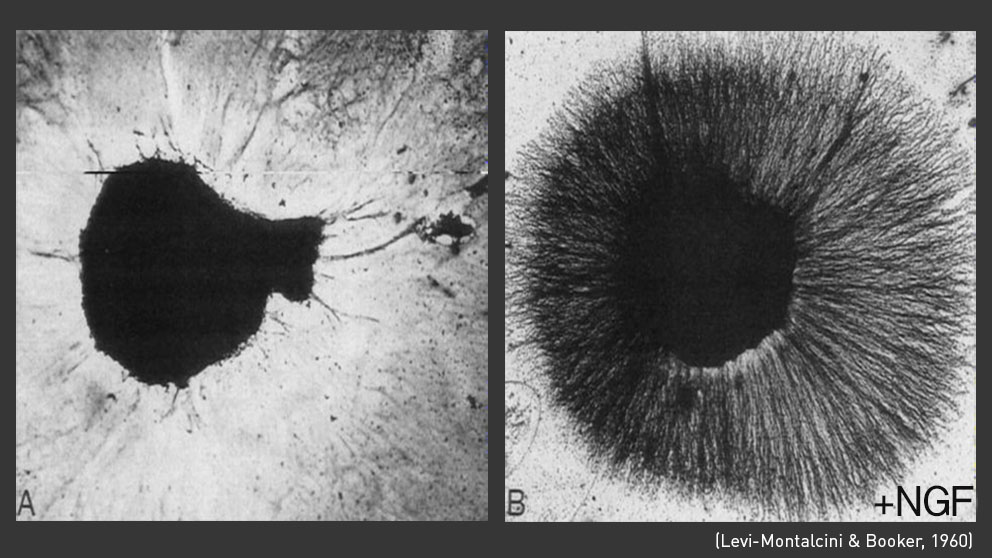
Levi-Montalcini and Cohen developed an antiserum to this secretion, which they used to block its function during mouse embryonic development. Strikingly, they found that treatment with this antiserum almost completely abolished sympathetic nerve development, comparable to the phenotype that resulted from ablating the wing bud in chick embryos. This was a remarkable breakthrough, as it conclusively showed that peripheral tissues secret a factor that directly influences neuronal survival in mammals.
Their discovery was published in 1960, and they termed the substance “Nerve Growth Factor,” or NGF. NGF was only the first of an entire class of chemotactic factors, later termed neurotrophins, which promote the growth and survival of specific subsets of neurons. A second function of NGF is to prune, or remove, nerve cells with poor connectivity. Levi-Montalcini observed this effect early on in her experiments, when she noted the high rate of cell death in early embryonic development. This “synaptic pruning” is essential for the development of the nervous system.
As the field of molecular neuroscience progressed it became evident that neurotrophins also have roles in the adult brain. They promote learning and memory via their influence on the survival of new synaptic transmissions. There is compelling evidence that decreased neurotrophic factors coincides with development of neurodegenerative disease, such as Alzheimer’s and Parkinson’s diseases, and these proteins are being actively researched as therapeutic tools for such diseases. In 1986 Rita Levi-Montalcini and Stanley Cohen shared the Nobel Prize in Physiology or Medicine, highlighting the importance of their work and the immeasurable effects it has had on multiple fields of scientific research.
Rita Levi-Montalcini had an incredible career, and NGF is only part of the story. She was an outspoken advocate for scientific funding and for women in science. In an interview with Scientific American in 1993, she explains, “I can do things that are very, very important, which I would never have been able to do if I did not receive it [the Nobel Prize]. It has given me the possibility of helping a lot of people.” She began her own foundation in 1992 with her sister, Paola, to provide counseling and mentors to children. In 2001 she expanded this foundation, which now provides educational support and scholarships to African women and children.
Italy made Levi-Montalcini a Senator for life in 2001, and in 2006 she had an infamous showdown with far right Italian politicians over a budget proposal that cut funding for research. (Yeah, she won that fight.) She continued to be an active participant in the research community, founding the European Brain Research Institute in 2002, and served as the Head of this Institute until her death in December 2012.
Rita Levi-Montalcini was one of the greatest scientific minds of the 20th century. She fought through deeply entrenched sexism and the staggering anti-Semitism of WWII to do the thing she loved most. The accolades and awards were never the goal. After learning that she won the Nobel Prize in 1986, she commented that, “It was a great honor. Still, there is no great a thrill as the moment of discovery.”
Ellen Elliott, Ph.D., is a postdoctoral fellow at The Jackson Laboratory for Genomic Medicine in Farmington, Conn. Ellen works in the laboratory of Adam Williams, Ph.D., where she is studying the function of long non-coding RNAs in TH2 cells and asthma. Follow Ellen on Twitter at @EllenNichole.