
Introduction
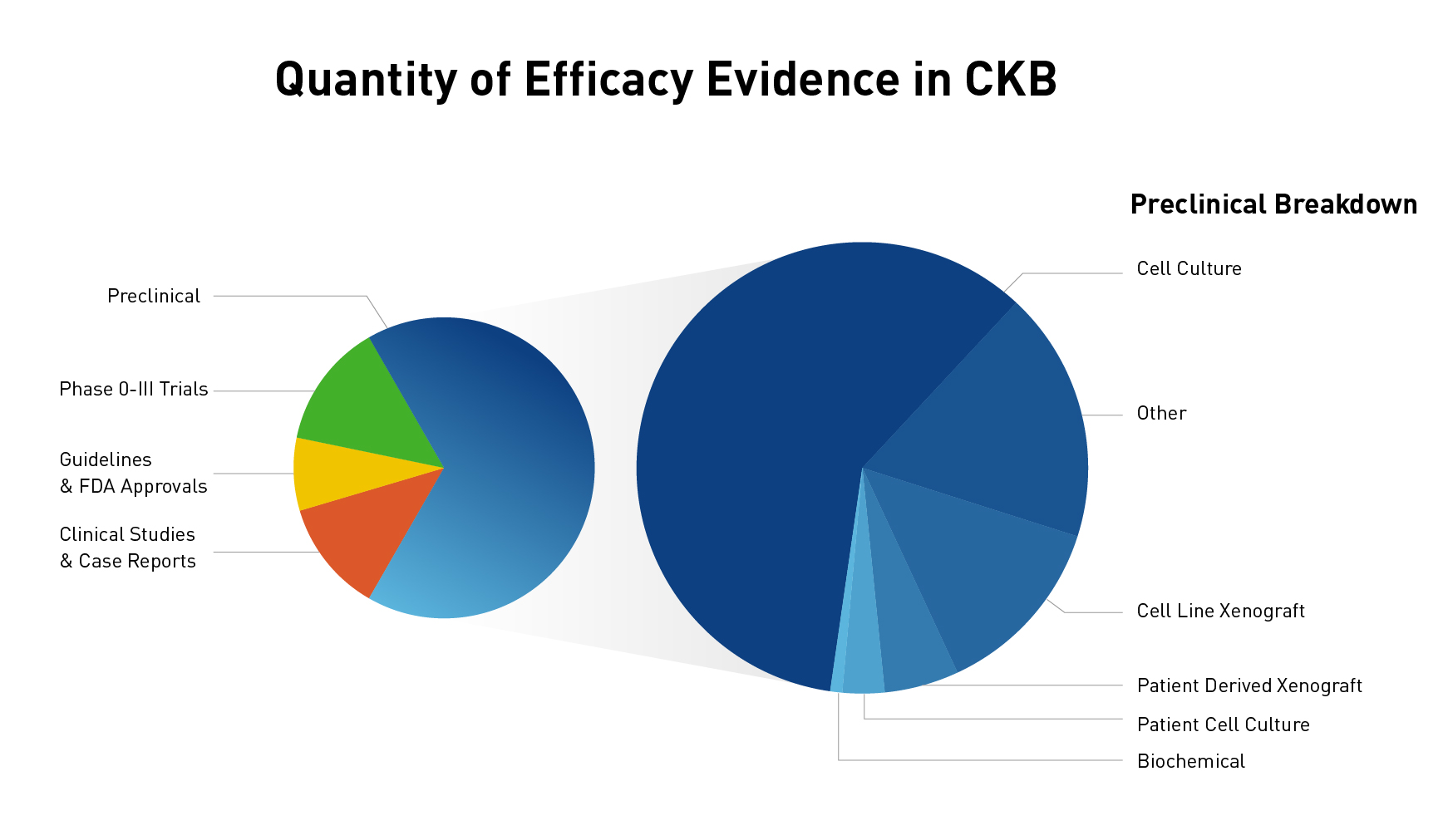
The JAX Clinical Knowledgebase (CKB) is a relational database that contains data associated with cancer variants, tumor types, and therapies. One of the unique features of CKB is efficacy evidence, which makes the connection between a molecular profile (one or more variants), therapy (one drug or multiple drugs used in combination), tumor type, and response. Utilizing peer-reviewed publications and abstracts, efficacy evidence is annotated as a brief one sentence summary of the study results, which allows you to quickly find answers to how a molecular profile responds to a therapy within a particular tumor type. With over 1800 genes, more than 40,000 variants, and nearly 30,000 lines of efficacy evidence, there is a wealth of data to explore. If you have visited CKB before, you may have explored some of the data curated from clinical trials, professional guidelines, clinical studies, and case reports (and if you haven’t visited, check out the link below). However, what you may not have realized is that we also have many lines of curated preclinical data (Figure 1). In fact, nearly two-thirds of our efficacy evidence lines are preclinical!
Figure 1: Quantity of clinical and preclinical data and break down of preclinical categories
What kind of preclinical data can you find in CKB?
As you can see in Figure 1, we curate preclinical study types ranging from biochemical studies to studies using patient-derived xenografts, and categorize their study type, or status, for easy filtering and sorting. When curating efficacy evidence, we include all the different types of data from a peer-reviewed publication and use the highest-level preclinical status possible. For example, if a paper used a lung cancer cell line to study the effects of an EGFR variant for a new drug and looked at both a biochemical assay and cell proliferation, we will include both types of evidence in a single line under the “Preclinical – Cell culture” status. However, if the authors had also engrafted the cell line in a mouse model, we would use the “Preclinical – Cell line xenograft”. You can see descriptions of the different preclinical categories we use in Figure 2.
Figure 2: Definitions of status types for preclinical data available in CKB
| CKB Preclinical Controlled Vocabulary | Definition |
|---|---|
| Cell Culture | A preclinical study conducted in established cell lines in culture |
| Cell line xenograft | A preclinical study conducted in cell line xenograft models |
| Patient cell culture | A preclinical study conducted in primary patient cells in culture |
| Patient-derived xenograft (PDX) | A preclinical study conducted in patient-derived xenograft (PDX) models |
| Biochemical | A preclinical study conducted biochemical analysis only, without cell proliferation, survival, or apoptosis data |
| Other | A preclinical study that cannot be classified as cell-culture, cell-line xenograft, patient cell-culture, or patient-derived xenograft (PDX). For example, a study conducted in a genetically engineered mouse model. |
What are some of the reasons you might be interested in preclinical data?
- Identify new drugs and combinations of drugs that are currently being tested preclinically. Although these therapies may not have made it to clinical trials, they may be promising new options for future clinical development.
- Identify possible resistance variants. Sometimes a variant is found to be present in a clinical sample following progressive disease, but is it driving the resistance? Using a controlled cell line expressing a presumed resistance variant and then assessing the level of drug resistance can help to confirm whether the variant may be predicted to cause resistance to a drug.
- Identify resistance mechanisms for drug development. If you are developing a new drug, it may be helpful to know the commonly observed resistance variants that could be relevant for development.
How can you search for preclinical efficacy evidence?
There are many ways you can find preclinical efficacy evidence within CKB. Often, you may come across efficacy evidence by following links – as we like to say, there are no dead ends in CKB. However, one method is to go to the Gene Detail page and click on the tab labeled “Gene Level Evidence”. All the columns are both sortable and filterable. The data is initially sorted by “Approval Status” with the highest-level approval (“FDA Approvals”) at the top. To find the preclinical data, type “preclinical” into the filter box above the “Approval Status” column. The data will then be filtered to show only preclinical efficacy evidence. This method of filtering will work regardless of how you get to efficacy evidence tab.
Summary
The JAX Clinical Knowledgebase is more than just a clinical database, it’s a valuable preclinical resource for research, clinical practice, and drug development. As part of the JAX mission to empower the global biomedical community, we continually work to improve and expand the CKB database to aid in our shared quest to improve human health.
And now we want to hear from YOU - tell us how you think preclinical data can support your research. Email CKBSupport@jax.org or tweet @JAX_CKB