Most efficient breeding scheme for generating Cre lox tissue-specific or inducible knockouts
eNews | September 23, 2011Simply put, the Cre/lox system rocks. It is one of the most powerful tools in the mouse geneticist’s toolbox. Why? Well, it enables them to generate tissue-specific and inducible knockouts and thereby have exquisite control over the location and timing of gene expression – important stuff when deleting a certain gene everywhere or during development leads to an embryonic lethal phenotype. And, it can be used to turn transgene expression on or off, track individual cells or cell lineages (as in the Brainbow or Confetti mice), generate inversions or translocations, and report gene expression. More novel and sophisticated uses for the Cre/lox system are being developed all the time.
Research mouse models that incorporate the Cre/lox system typically involve generating double, triple, quadruple, or even n-tuple (is that really a word?) mutant/transgenic mice. As you can imagine, breeding such mice can get a bit complicated. Our Technical Information Services (TIS) is receiving more and more requests for help in designing breeding schemes for producing Cre/lox mice. Explaining these schemes can be difficult, and I swear that I can sometimes feel customers’ eyes cross while I’m trying to explain them over the telephone. So, in this post, I’m outlining a typical breeding scheme that I hope will help demystify the process of using the Cre/lox system to generate tissue-specific or inducible knockout mice.
Knockouts: remember, one cross just isn’t enough!
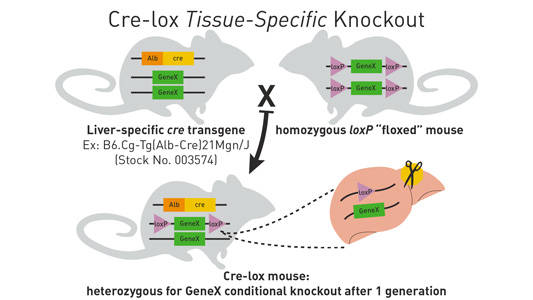
Here is the most efficient breeding scheme for generating Cre/lox tissue-specific or inducible knockouts. To generate mice that are heterozygous for a loxP-flanked allele and hemizygous/heterozygous for the cre transgene, mate a homozygous loxP-flanked mouse of interest to a cre transgenic mouse strain (see Figure 1 below). Approximately 50% of the offspring will be heterozygous for theloxP allele and hemizygous/heterozygous for the cre transgene.
Figure 1
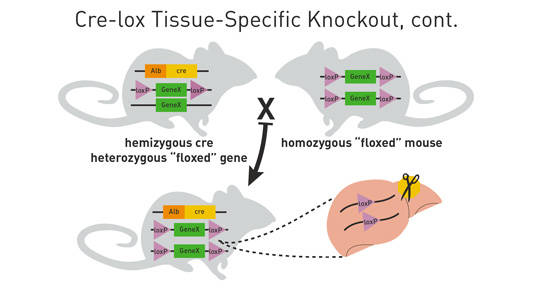
Mate these mice back to the homozygous loxP-flanked mice (see Figure 2 below). Approximately 25% of the progeny from this mating will be homozygous for theloxP-flanked allele and hemizygous/heterozygous for the cre transgene. These will be your experimental mice.
Figure 2
About 25% of the offspring from this mating will be homozygous for theloxP flanked allele but will have no cre transgene. Depending on your experimental parameters, you can use these as controls (see Figure 3A below). Because the controls will not have Cre recombinase, any phenotypic differences between them and the Cre/lox mice should be due to the deleted gene in the Cre/lox mice. If appropriate for your experiments, you can maintain a colony that is homozygous for the loxP flanked allele, but in which one parent was hemizygous/heterozygous for cre and the other was a noncarrier/wild-type for cre.
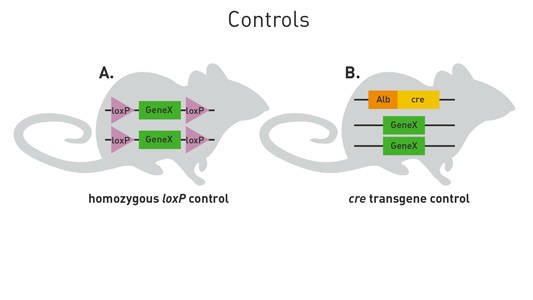
This breeding scheme may not be the most efficient one for producing all Cre/lox strains. You will have to adapt it to the genotypes you want to produce and the genetic backgrounds and characteristics of the loxP-flanked and cre strains you use. To verify that Cre recombinase expression does not contribute to a phenotype of interest, I suggest using the cre strain itself (without any loxP-flanked alleles) as a control (Figure 3B). One of my colleagues, Andy, has written an awesome blog post on how Cre recombinase can produce a phenotype on its own. I highly recommend that you check out his blog.
Figure 3
The use of the Cre/loxP system for generating tissue specific or inducible knockouts is a powerful tool for mouse genetics, but it is only one piece of the total picture.
